
The air bags in automobiles were once inflated by nitrogen gas generated by the rapid decomposition of
sodium azide, NaN3.
2 NaNz (s) 2 Na (s) + 3 N2 (g)
If an air bag has a volume of 53.4 L and is to be filled with nitrogen gas at a pressure of 1.07 atm and a
temperature of 23.7°C, how many moles of NaNz must decompose? You may assume the Ny behaves as
an ideal gas.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
The air bags in automobiles were once inflated by nitrogen gas generated by the rapid decomposition...
Questions







Mathematics, 21.04.2020 19:00

History, 21.04.2020 19:00


Mathematics, 21.04.2020 19:00

Spanish, 21.04.2020 19:00




Mathematics, 21.04.2020 19:00





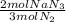 = 1.57 mol NaN₃
= 1.57 mol NaN₃

