Chemistry, 13.04.2021 03:40 ridzrana02
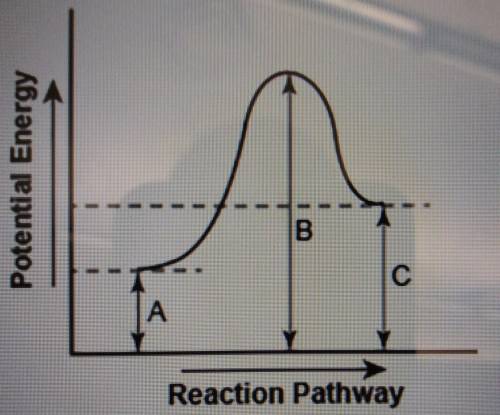
The diagram shows the potential energy changes for a reaction pathway. (10 points) Potential Energy B C TA Reaction Pathway Part 1: Does the diagram illustrate an endothermic or an exothermic reaction? Give reasons in support of your answer. Part 2: Describe how you can determine the total change in enthalpy and activation energy from the diagram and if each is positive or negative.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
The diagram shows the potential energy changes for a reaction pathway. (10 points) Potential Energy...
Questions


Social Studies, 06.07.2019 09:30

History, 06.07.2019 09:30


English, 06.07.2019 09:30

English, 06.07.2019 09:30





English, 06.07.2019 09:30

English, 06.07.2019 09:30

Mathematics, 06.07.2019 09:30

Biology, 06.07.2019 09:30


History, 06.07.2019 09:30



Mathematics, 06.07.2019 09:30