Chemistry, 13.04.2021 02:10 Misspaige4453
It is time to practice using potential energy diagrams. Respond to the three questions below on energy diagrams and submit to your instructor.
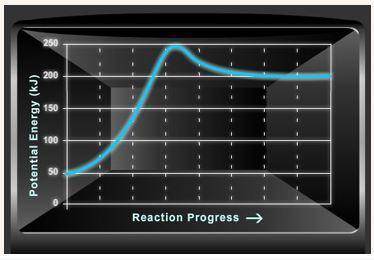
Consider the potential energy diagram shown below. This graph shows the chemical potential energy in a reaction system over time. The y-axis is potential energy in kilojoules. The x-axis is the reaction progress, or time.
Does this graph represent an endothermic or an exothermic reaction? Explain your answer.
What is the enthalpy change, ΔH, for this reaction? Show your work.
What is the activation energy, Ea, for this reaction? Show your work.
In a particular chemical reaction, the energy of the reactants is 30 kJ and the energy of the products is 5 kJ. The maximum energy of the system is 40 kJ.
Sketch a potential energy diagram for this reaction. Make sure to label the energy of the reactants, the energy of the products, the activation energy, and the enthalpy change for the reaction.
What is the activation energy for this reaction?
What is the enthalpy change for this reaction?
Is this reaction endothermic or exothermic? Explain your answer in two ways: first, using the energy values, and second, by referring to the shape of the graph.
The coating on the head of a match is highly flammable. When it burns, it releases a great deal of energy. However, before the match can burn, it must gain a small amount of energy from a spark. That spark is typically produced by striking (rubbing) the match head against a rough surface. Sketch and describe a potential energy diagram that represents the striking and burning of the match. Remember to label the diagram with the energy changes that occur. Your answer must include the potential energy diagram and a written description. (Note: you do not have to use actual energy values.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1
You know the right answer?
It is time to practice using potential energy diagrams. Respond to the three questions below on ener...
Questions

History, 09.12.2020 01:30



Business, 09.12.2020 01:30

Chemistry, 09.12.2020 01:30

English, 09.12.2020 01:30



Spanish, 09.12.2020 01:30

English, 09.12.2020 01:30

Chemistry, 09.12.2020 01:30

Chemistry, 09.12.2020 01:30



Health, 09.12.2020 01:30


Mathematics, 09.12.2020 01:30


Mathematics, 09.12.2020 01:30