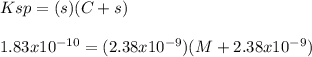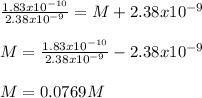Chemistry, 11.04.2021 23:20 xeskimopie
What is the molarity of NaCl in which AgCl has a molar solubility of 2.38 x 10-9 mol /L? The Ksp for Silver Chloride is: 1.83 x 10-10.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2
You know the right answer?
What is the molarity of NaCl in which AgCl has a molar solubility of 2.38 x 10-9 mol /L? The Ksp for...
Questions

Mathematics, 19.05.2021 18:00

Mathematics, 19.05.2021 18:00

History, 19.05.2021 18:00

Mathematics, 19.05.2021 18:00

Mathematics, 19.05.2021 18:00

Mathematics, 19.05.2021 18:00

Mathematics, 19.05.2021 18:00


Mathematics, 19.05.2021 18:00


Mathematics, 19.05.2021 18:00

Physics, 19.05.2021 18:00




Computers and Technology, 19.05.2021 18:00

Physics, 19.05.2021 18:00


Mathematics, 19.05.2021 18:00

![AgCl(s)\rightleftharpoons Ag^+(aq)+Cl^-(aq)\\\\Ksp=[Ag^+][Cl^-]\\](/tpl/images/1251/6223/6391c.png)