How many moles are in 50.00 grams of CH4 ? Watch your significant
figures.
3.1 g CH4
3....

Chemistry, 09.04.2021 21:10 sabrinarasull1pe6s61
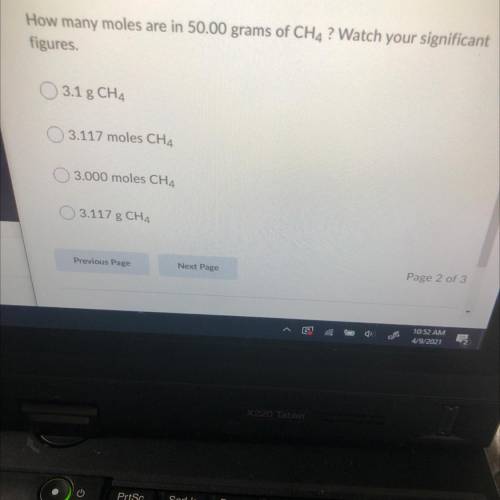
How many moles are in 50.00 grams of CH4 ? Watch your significant
figures.
3.1 g CH4
3.117 moles CH4
3.000 moles CH4
3.117 g CH4

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 06:40
1.) which of the following is a molecule but not a compound? a.he b.f2 c.h2o d.ch4 2.) what is a physical combination of substances? a.a compound b.a molecule c.a mixture d.an element 3.) what is a chemical combination of substances? a.a compound b.an atom c.a mixture d.an element 4.) what is the relationship between the solute and solvent in a solution? a.they form a compound b.they form a mixture c.they form molecules d.they form chemical bonds 5.) the gases in air dissolve in water. what would be one way to reduce the amount of a gas dissolved in water? a.add more water b.reduce the air pressure c.increase the air pressure d.stir the water 6.) how would you determine the solubility of a substance? a.find how well it dissolved various substances. b.find the mass and the volume of the substance. c.find the temperature at which the substance evaporated. d.find how much i was able to dissolve in a solute. 7.) the periodic table organizes all of the kinds of a.molecules. b.compounds. c.atoms. d.ions. 8.)what distinguishes two substances combined to become a compound vs. two substances combined to become a mixture? a.whether they can be easily separated b.whether they chemically bond together c.whether they both are visible d.whether they are heterogeneous 9.) the principle components of air are: n2 78% o2 21% ar 0.95% co2 0.038% this is a solution of a.molecules and atoms. b.molecules. c.compounds and molecules. d.atoms.
Answers: 1

Chemistry, 23.06.2019 15:20
How many stereoisomers will be formed from the addition of phenyllithium to this molecule?
Answers: 1

Chemistry, 24.06.2019 03:30
Concentrated aqueous nitric acid is 70.4 wt% hno3 and has a concentration of 15.9 m. calculate the volume of concentrated nitric acid that should be diluted to 1.80 l to form a 5.00 m hno3 solution.
Answers: 2
You know the right answer?
Questions

Mathematics, 09.07.2019 10:00

English, 09.07.2019 10:00

Mathematics, 09.07.2019 10:00

Biology, 09.07.2019 10:00


Mathematics, 09.07.2019 10:00




History, 09.07.2019 10:00


Mathematics, 09.07.2019 10:00

History, 09.07.2019 10:00

Mathematics, 09.07.2019 10:00

Mathematics, 09.07.2019 10:00


Mathematics, 09.07.2019 10:00





