
Chemistry, 08.04.2021 18:40 silviamgarcia
A reaction is first order with respect to reactant A and second order with respect to reactant B. Starting with [A] = 0.175 M and [B] = 0.00250 M, the reaction rate is 2.83 x 10−5 M. S−1. What is the rate constant k? Show your work.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
A reaction is first order with respect to reactant A and second order with respect to reactant B. St...
Questions





English, 15.01.2020 06:31


Mathematics, 15.01.2020 06:31

History, 15.01.2020 06:31


English, 15.01.2020 06:31

Mathematics, 15.01.2020 06:31


English, 15.01.2020 06:31

Mathematics, 15.01.2020 06:31

Mathematics, 15.01.2020 06:31


Mathematics, 15.01.2020 06:31

Mathematics, 15.01.2020 06:31


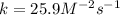
![r=k[A][B]^2](/tpl/images/1246/4928/487f0.png)
![k=\frac{r}{[A][B]^2} \\\\k=\frac{2.83x10^{-5}M/s}{(0.175M)(0.00250M)^2}\\\\k=25.9M^{-2}s^{-1}](/tpl/images/1246/4928/29bac.png)



