

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1

Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1
You know the right answer?
A buffer is made with 0.493 M of the weak base methylamine () with 0.493 M (KA = 5.6 x 10^-4)
What...
Questions

Advanced Placement (AP), 23.02.2021 19:10




Mathematics, 23.02.2021 19:10

SAT, 23.02.2021 19:10

Mathematics, 23.02.2021 19:10








History, 23.02.2021 19:10





Physics, 23.02.2021 19:10

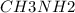 ) with 0.493 M
) with 0.493 M 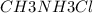 (KA = 5.6 x 10^-4)
What is the pH of this buffer system?
(KA = 5.6 x 10^-4)
What is the pH of this buffer system?

