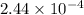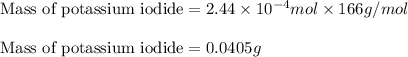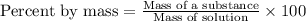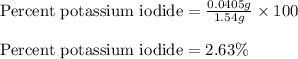Chemistry, 06.04.2021 05:10 jjimenez0276
The iodide in a sample that also contained chloride was converted to iodate by treatment with an excess of bromine: The unused bromine was removed by boiling; an excess of barium ion was then added to precipitate the iodate: In the analysis of a 1.54-g sample, 0.0596 g of barium iodate was recovered. Express the results of this analysis as percent potassium iodide.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
The iodide in a sample that also contained chloride was converted to iodate by treatment with an exc...
Questions

Mathematics, 11.12.2020 05:40

Mathematics, 11.12.2020 05:40




Mathematics, 11.12.2020 05:40


Mathematics, 11.12.2020 05:40



History, 11.12.2020 05:40

World Languages, 11.12.2020 05:40

Social Studies, 11.12.2020 05:40

History, 11.12.2020 05:40

History, 11.12.2020 05:40


Mathematics, 11.12.2020 05:40


Mathematics, 11.12.2020 05:40

Physics, 11.12.2020 05:40

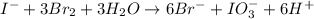 (i)
(i)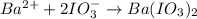 ......(ii)
......(ii)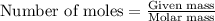
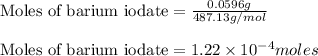
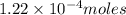 of barium iodate will be produced by
of barium iodate will be produced by 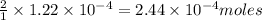 of iodate ions
of iodate ions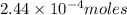 of iodate ions will be produced by
of iodate ions will be produced by 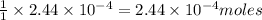 of iodine ions
of iodine ions