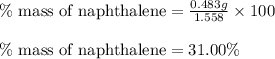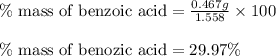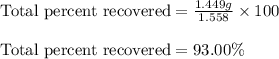Chemistry, 06.04.2021 05:00 aileenf598
Data Collection
Mass of the original sample of mixture (g) 1.558
Mass of recovered naphthalene (g) 0.483
Mass of recovered 3-nitroaniline (g) 0.499
Mass of recovered benzoic acid (g) 0.467
Calculations:
a. % by mass of naphthalene in original sample.
b. % by mass of 3-nitroaniline in original sample.
c. % by mass of benzoic acid in original sample.
d. total percent recovered.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1


Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
You know the right answer?
Data Collection
Mass of the original sample of mixture (g) 1.558
Mass of recovered naphthale...
Mass of recovered naphthale...
Questions

Mathematics, 11.09.2020 01:01

Mathematics, 11.09.2020 01:01

Mathematics, 11.09.2020 01:01

Mathematics, 11.09.2020 01:01

Mathematics, 11.09.2020 01:01

Mathematics, 11.09.2020 01:01

Mathematics, 11.09.2020 01:01

Mathematics, 11.09.2020 01:01

Mathematics, 11.09.2020 01:01

Mathematics, 11.09.2020 01:01

Physics, 11.09.2020 01:01

Mathematics, 11.09.2020 01:01

Mathematics, 11.09.2020 01:01

Mathematics, 11.09.2020 01:01

World Languages, 11.09.2020 01:01

Geography, 11.09.2020 01:01

Mathematics, 11.09.2020 01:01

Mathematics, 11.09.2020 01:01

Mathematics, 11.09.2020 01:01

Mathematics, 11.09.2020 01:01

 ......(1)
......(1)