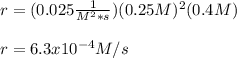Study the reaction and read the statement.
Xe+3F2→XeF6
The rate constant for this reaction is...

Study the reaction and read the statement.
Xe+3F2→XeF6
The rate constant for this reaction is 0.025, and the reaction is second order in Xe and first order in F2.
What is the rate of the reaction if [Xe] is 0.25 M, and [F2] is 0.4 M?
4.0 × 10–^4
2.5 × 10–^3
1.5 × 10–^3
6.3 × 10–^4

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
Questions









History, 25.02.2020 17:00

Mathematics, 25.02.2020 17:00

History, 25.02.2020 17:00

Mathematics, 25.02.2020 17:00




Biology, 25.02.2020 17:00



Mathematics, 25.02.2020 17:00

![r=k[Xe]^2[F_2]](/tpl/images/1238/0471/c5483.png)