
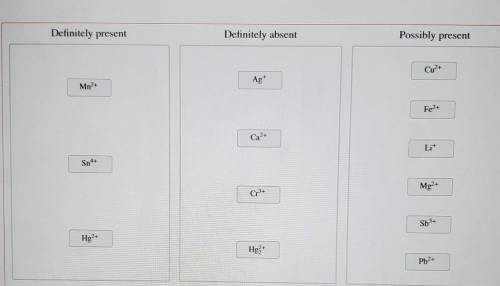
A solution containing a mixture of metal cations was treated with dilute HCI and no precipitate formed. Next, HS was bubbled through the acidic solution. A precipitate formed and was filtered off. Then, the pH was raised to about 8 and HS was again bubbled through the solution. A precipitate again formed and was filtered off. Finally, the solution was treated with a sodium carbonate solution, which resulted in no precipitation.
Classify the metal ions based on whether they were definitely present, definitely absent, or whether it is possible they were present in the original mixture.
Mn2+
Sn4+
Hg2+
Ag+
Ca2+
Cr3+
Hg2 2+
Cu2+
Fe2+
Li+
Mg2+
Sb3+
Pb2+

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:40
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 01:30
Follow the steps provided in the simulation to add water to the graduated cylinder, select one of the three samples (copper, silver, or gold), set its mass to the values given in the statements below, and calculate its density. here is a summary of the steps required: add water by clicking and holding prepare a known volume of water button. until the desired volume of water has been added. if more than the desired volume is added, click the reset button. button and redo the procedure. a single click will add about 21.0 ml of water. to set the mass, click and hold weigh out metal button. until the desired amount of metal is added to the weighing pan. once the desired mass of the metal is added, release the button. transfer the metal to water and then click on calculate density button. to see how the density is calculated using water displacement to measure the volume of the solid. to save time you can approximate the initial volume of water to â±1 ml and the initial mass of the solid to â±1 g. for example, if you are asked to add 23 ml of water, add between 22 ml and 24 ml. which metals in each of the following sets will have equal density? check all that apply.
Answers: 1

You know the right answer?
A solution containing a mixture of metal cations was treated with dilute HCI and no precipitate form...
Questions




Mathematics, 18.03.2021 20:50




Mathematics, 18.03.2021 20:50


Biology, 18.03.2021 20:50

Mathematics, 18.03.2021 20:50

Mathematics, 18.03.2021 20:50

Mathematics, 18.03.2021 20:50


English, 18.03.2021 20:50




History, 18.03.2021 20:50

Mathematics, 18.03.2021 20:50



