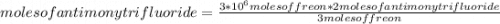Chemistry, 27.03.2021 07:00 zacksoccer8279
Freon, a very useful refrigerant, is produced in the following reaction:
3CCl4 (g) + 2SbF3 (s) → 3CCl2F2 (g) + 2 SbCl3 (s)
If a chemist wants to make 3.0 x 106 moles of Freon using excess carbon tetrachloride, how many moles of antimony trifluoride will the chemist need?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3
You know the right answer?
Freon, a very useful refrigerant, is produced in the following reaction:
3CCl4 (g) + 2SbF3 (s) → 3C...
Questions


Mathematics, 24.06.2021 05:30



Mathematics, 24.06.2021 05:30

Spanish, 24.06.2021 05:30

Mathematics, 24.06.2021 05:30


Biology, 24.06.2021 05:30


Social Studies, 24.06.2021 05:30

Mathematics, 24.06.2021 05:30

Mathematics, 24.06.2021 05:30

Mathematics, 24.06.2021 05:30



Mathematics, 24.06.2021 05:30

Mathematics, 24.06.2021 05:30

Spanish, 24.06.2021 05:30

Mathematics, 24.06.2021 05:40