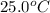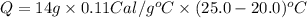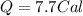Chemistry, 16.10.2019 15:30 oofoofoof1
Suppose a 14-gram sample of iron is heated from 20.0°c to 25.0°c. the specific heat of iron is 0.11 cal/g°c. how much heat energy was absorbed by the iron? 38.5 cal 7.7 cal 636 cal 69.3 cal

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3


Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3
You know the right answer?
Suppose a 14-gram sample of iron is heated from 20.0°c to 25.0°c. the specific heat of iron is 0.11...
Questions


Mathematics, 27.01.2021 21:20

Mathematics, 27.01.2021 21:20


Mathematics, 27.01.2021 21:20


Mathematics, 27.01.2021 21:20

Chemistry, 27.01.2021 21:20

Social Studies, 27.01.2021 21:20



Chemistry, 27.01.2021 21:20



Mathematics, 27.01.2021 21:20



Mathematics, 27.01.2021 21:20

History, 27.01.2021 21:20

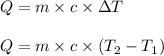
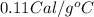
 = change in temperature
= change in temperature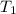 = initial temperature =
= initial temperature = 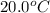
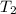 = final temperature =
= final temperature =