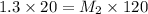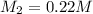Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1

Chemistry, 23.06.2019 11:20
The chemical composition of soil varies with depth. an article in communications in soil science and plant analysis describes chemical analyses of soil taken from a farm in western australia. fifty specimens were each taken at depths 50 and 250 cm. at a depth of 50 cm, the average no3 concentration (in mg/l) was 88.5 with a standard deviation of 49.4. at a depth of 250 cm, the average concentration was 110.6 with a standard deviation of 51.5. find a 95% confidence interval for the difference in no3 concentrations at the two depths.
Answers: 1
You know the right answer?
(On the verge of tears)
Calculate the final molarity of a 20mL- 1.3M salt solution after it has bee...
Questions

History, 03.07.2021 16:40


English, 03.07.2021 16:40

Social Studies, 03.07.2021 16:40



Mathematics, 03.07.2021 16:40

English, 03.07.2021 16:40

Health, 03.07.2021 16:40

Social Studies, 03.07.2021 16:40

Mathematics, 03.07.2021 16:40

Mathematics, 03.07.2021 16:40

Mathematics, 03.07.2021 16:40


Mathematics, 03.07.2021 16:40

Health, 03.07.2021 16:40

Mathematics, 03.07.2021 16:40

Mathematics, 03.07.2021 16:40


Biology, 03.07.2021 16:50

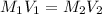
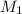 = molarity of stock solution = 1.3 M
= molarity of stock solution = 1.3 M
 = volume of stock solution = 20 ml
= volume of stock solution = 20 ml