The information below describes a redox reaction.
Ag+ (aq) + Al(s) -> Ag(s) + Al^3+ (aq)
<...

Chemistry, 25.03.2021 22:10 iesps010411
The information below describes a redox reaction.
Ag+ (aq) + Al(s) -> Ag(s) + Al^3+ (aq)
Ag+ (aq) + e^- -> Ag(s)
Al(s) -> Al^3+ (aq) + 3e^-
What is the coefficient of silver in the final, balanced equation for this reaction?
A. 1
B. 2
C. 3
D. 4
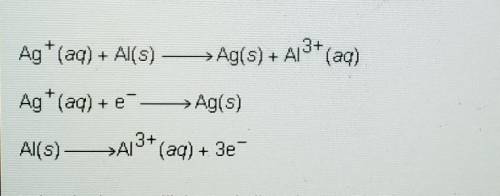

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2


Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
Questions

Biology, 01.04.2021 21:30

Arts, 01.04.2021 21:30

Mathematics, 01.04.2021 21:30






English, 01.04.2021 21:30

Mathematics, 01.04.2021 21:30

English, 01.04.2021 21:30

Computers and Technology, 01.04.2021 21:30


Mathematics, 01.04.2021 21:30




Chemistry, 01.04.2021 21:30

Mathematics, 01.04.2021 21:30

History, 01.04.2021 21:30



