
Chemistry, 25.03.2021 05:10 gabriel5575
PLEASE HELP
1. A gas with a volume of 4.0 L at a pressure of 2.03 atm is allowed to expand to a volume of 12.0 L. What is the pressure in atm in the container if the temperature remains constant. Please show work (including the equation you used).
2.The volume of a gas is 200 mL at 350.0 kPa pressure. What will the volume be when the pressure is reduced to 120.0 kPa, assuming the temperature remains constant. Please show all work for full credit. Please show work (including the equation you used).
3.What is the original pressure of a bike tire if the current pressure is 150 atm and the volume changed from 1000 mL to 700 mL. Please show work (including the equation you used).

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1

Chemistry, 23.06.2019 06:30
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1

Chemistry, 23.06.2019 09:20
Which of the following occurs along coasts during the day?
Answers: 3
You know the right answer?
PLEASE HELP
1. A gas with a volume of 4.0 L at a pressure of 2.03 atm is allowed to expand to a vol...
Questions


Spanish, 26.01.2021 01:40

Mathematics, 26.01.2021 01:40

Biology, 26.01.2021 01:40

History, 26.01.2021 01:40


Mathematics, 26.01.2021 01:40


Spanish, 26.01.2021 01:40

Mathematics, 26.01.2021 01:40


Physics, 26.01.2021 01:40


Mathematics, 26.01.2021 01:40


English, 26.01.2021 01:40

Mathematics, 26.01.2021 01:40


Geography, 26.01.2021 01:40

Mathematics, 26.01.2021 01:40

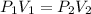 (also called Boyle's Law), where P₁ and V₁ denote the initial pressure and volume, and P₂ and V₂ denote the final (or changed) pressure and volume, respectively.
(also called Boyle's Law), where P₁ and V₁ denote the initial pressure and volume, and P₂ and V₂ denote the final (or changed) pressure and volume, respectively.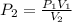 .
. ![\[P_2=\frac{\left ( 2.03 \text{ atm} \right )\left ( 4.0 \text{ L} \right )}{\left ( 12.0 \text{ L} \right )}\] = 0.67 atm.](/tpl/images/1219/2359/100f6.png)
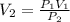
![\[V_2=\frac{\left ( 350.0 \text{ kPa} \right )\left ( 200 \text{ mL} \right )}{\left ( 120.0 \text{ kPa} \right )}\] = 583 mL.](/tpl/images/1219/2359/ee8b8.png)
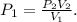
![\[P_1=\frac{\left ( 150 \text{ atm} \right )\left ( 700 \text{ mL} \right )}{\left ( 1000 \text{ mL} \right )}\] = 105 atm.](/tpl/images/1219/2359/a3484.png)


