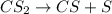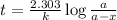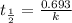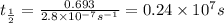Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
The decomposition of carbon disulfide, CS2, to carbon monosulfide, CS, and sulfur is first order wit...
Questions

History, 11.10.2020 09:01



Engineering, 11.10.2020 09:01


Mathematics, 11.10.2020 09:01



Mathematics, 11.10.2020 09:01



Arts, 11.10.2020 09:01

Mathematics, 11.10.2020 09:01



Social Studies, 11.10.2020 09:01




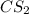 , to carbon monosulfide, CS, and sulfur is first order with k =
, to carbon monosulfide, CS, and sulfur is first order with k = 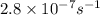 at 1000oC.
at 1000oC.