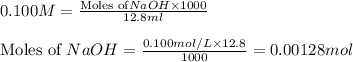Chemistry, 22.03.2021 22:30 jayden6467
molecular formula citric acid2.A 10.0 mL sample of pineapple juice was titrated with 0.100 M sodium hydroxide solution. The average volume of NaOH required to reach the endpoint was 12.8 mL. a. Calculate the number of moles of sodium hydroxide required to reach the endpoint. Show your work in equation editor. Remember to use units and report your answer to the proper number of significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 23:00
Movement that is like a t a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. transfers energy from one location to another a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1
You know the right answer?
molecular formula citric acid2.A 10.0 mL sample of pineapple juice was titrated with 0.100 M sodium...
Questions

Biology, 15.07.2019 19:30

Social Studies, 15.07.2019 19:30

Mathematics, 15.07.2019 19:30

Mathematics, 15.07.2019 19:30

Social Studies, 15.07.2019 19:30

Mathematics, 15.07.2019 19:30



Chemistry, 15.07.2019 19:30

Social Studies, 15.07.2019 19:30


Chemistry, 15.07.2019 19:30

Chemistry, 15.07.2019 19:30




Mathematics, 15.07.2019 19:30

Biology, 15.07.2019 19:30

Social Studies, 15.07.2019 19:30

Biology, 15.07.2019 19:30

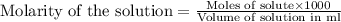
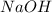 solution = 0.100 M
solution = 0.100 M