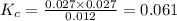Phosphorous pentachloride decomposes according to the reaction
PCl5(g)=PCl3(g)+Cl2(g)
A 12.3 g sample of PCl5 is added to a sealed 1.50 L flask and the reaction is allowed to come to equilibrium at a constant temperature. At equilibrium, 31.8% of the PCl5 remains. What is the equilibrium constant, Kc, for the reaction?
Question is asking for Kc

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:00
19. at high pressures, how does the volume of a real gas compare with the volume of an ideal gas under the same conditions, and why? eman- it is much less because real gas partides are not moving. there is no difference because the gas laws are always obeyed. it is much less because at high pressures the temperature drops. it is much greater because real gas partides take up space.
Answers: 1

Chemistry, 21.06.2019 17:30
What is the percentage by mass of silicon (si) in iron aluminum silicate (fe3al2(sio4)3)?
Answers: 2

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
You know the right answer?
Phosphorous pentachloride decomposes according to the reaction
PCl5(g)=PCl3(g)+Cl2(g)
A...
A...
Questions


Mathematics, 11.05.2020 10:57

English, 11.05.2020 10:57





Biology, 11.05.2020 10:57

English, 11.05.2020 10:57




Social Studies, 11.05.2020 10:57



Computers and Technology, 11.05.2020 10:57

History, 11.05.2020 10:57

Mathematics, 11.05.2020 10:57

History, 11.05.2020 10:57

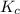 , for the reaction is 0.061.
, for the reaction is 0.061.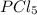 =
= 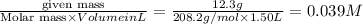
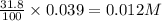
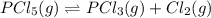
![K_c=\frac{[Cl_2]\times [PCl_3]}{[PCl_5]}](/tpl/images/1209/0088/ffe89.png)