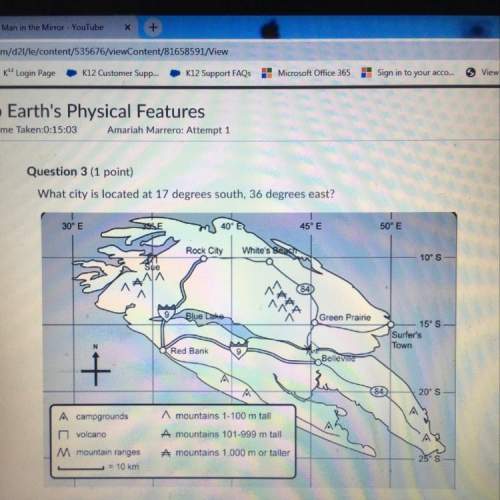
Chemistry, 20.03.2021 01:00 genyjoannerubiera
Look at the diagram below that shows the reaction quotiant, Q for this reaction. A(s) + B(g) + 2C(aq) 2D(g) + E(aq) The reaction begins at equilibrium. There are 6 processes listed below that are applied individually to put a stress on the equilibrium. For each process, identify the position of Q that occurs immediately after the stress is applied. Then drag the purple number that identifies the process to the proper gray boxes in the graph. There can be more than 1 number in each gray box. The line marked K shows the initial equilibrium. 2 Some B is removed 4 Some E is removed Some A is added 3 Some D is added 5 Water is added. Gas volume does not change. 6 The container volume of the gases is increased

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
You know the right answer?
Look at the diagram below that shows the reaction quotiant, Q for this reaction. A(s) + B(g) + 2C(aq...
Questions



Mathematics, 22.01.2021 21:40

Mathematics, 22.01.2021 21:40





Mathematics, 22.01.2021 21:40

Mathematics, 22.01.2021 21:40


Mathematics, 22.01.2021 21:40

History, 22.01.2021 21:40


English, 22.01.2021 21:40

English, 22.01.2021 21:40

Mathematics, 22.01.2021 21:40

Mathematics, 22.01.2021 21:40