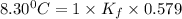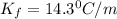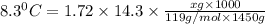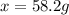Chemistry, 18.03.2021 01:20 Drevei6969
When 74.8g of alanine C3H7NO2 are dissolved in 1450.g of a certain mystery liquid X, the freezing point of the solution is 8.30°C less than the freezing point of pure X. Calculate the mass of potassium bromide that must be dissolved in the same mass of X to produce the same depression in freezing point. The van't Hoff factor =i1.72 for potassium bromide in X.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
When 74.8g of alanine C3H7NO2 are dissolved in 1450.g of a certain mystery liquid X, the freezing po...
Questions

Mathematics, 22.06.2019 22:00




Mathematics, 22.06.2019 22:00

Mathematics, 22.06.2019 22:00



History, 22.06.2019 22:00

English, 22.06.2019 22:00


Business, 22.06.2019 22:00




Mathematics, 22.06.2019 22:00


Mathematics, 22.06.2019 22:00


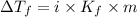
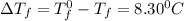 = Depression in freezing point
= Depression in freezing point
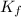 = freezing point constant =
= freezing point constant =