a. neutrons and electrons

Chemistry, 01.01.2020 00:31 ayaanwaseem
1. which two particles are found in the nucleus of an atom?
a. neutrons and electrons
b. protons and electrons
c. protons and neutrons
d. neutrons and atoms
2. the table below gives the atomic mass and relative abundance values for the three isotopes of element m.
what is the average atomic mass (in amu) of element m?
a.2.86
b.5.36
c.24.30
d.24.98
relative abundance (%) atomic mass (amu)
78.99 23.9850
10.00 24.9858
11.01 25.9826
3. a particle that orbits the nucleus in an atom is called a .
4. the table below gives the numbers of protons, electrons, and neutrons in four atoms.
atom number of protons number of neutrons number of electrons
1 9 8 8
2 9 9 8
3 9 9 10
4 9 10 9
based on the table, which atom has a charge of –1?
a.1
b.2
c.3
d.4
5. boron occurs naturally as two isotopes. what is the difference between these isotopes?
a. they have different numbers of electrons and different charges.
b. they have different numbers of neutrons and different charges.
c. they have different numbers of protons and different mass numbers.
d. they have different numbers of neutrons and different mass numbers.
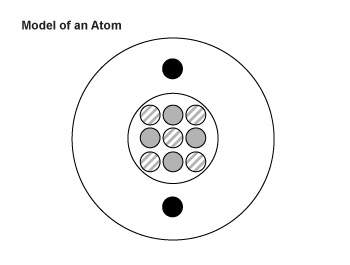
6. the diagram below is an artist’s impression of a single atom of element be. the neutrons are shown with stripes, the protons are gray, and the electrons are black. these particles are not drawn to scale.
which element below could be an isotope of this atom?
a. sodium-10
b. beryllium-10
c. boron-9
d. carbon-9
7. the mass number of fe2+ is 56. how many neutrons are there in a single fe2+ atom?
a. 28
b. 30
c. 56
d. 58
8. a scientist uses an accelerator and high energy electrons to study the particles inside the protons of a helium atom. what particles is the scientist studying?
a. the he neutrons
b. the he nucleus
c. the he electrons
d. the he quarks
9. which element has 16 protons in its nucleus?
a. neon
b. oxygen
c. sodium
d. sulfur
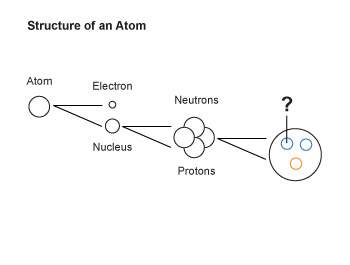
10. the diagram below shows some subatomic particles.
what is the particle that is labeled with a question mark in the diagram?
a. beta particle
b. quark
c. isotope
d. alpha particle


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
1. which two particles are found in the nucleus of an atom?
a. neutrons and electrons
a. neutrons and electrons
Questions


Spanish, 02.04.2021 03:40

Biology, 02.04.2021 03:40

English, 02.04.2021 03:40


English, 02.04.2021 03:40

Social Studies, 02.04.2021 03:40

Computers and Technology, 02.04.2021 03:40

Mathematics, 02.04.2021 03:40

Mathematics, 02.04.2021 03:40

English, 02.04.2021 03:40

Arts, 02.04.2021 03:40

Biology, 02.04.2021 03:40



Chemistry, 02.04.2021 03:40

Biology, 02.04.2021 03:40

Business, 02.04.2021 03:40

Mathematics, 02.04.2021 03:40



