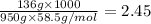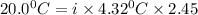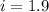Chemistry, 12.03.2021 15:30 Cnolteb5663
When 136g of glycine are dissolved in of a certain mystery liquid , the freezing point of the solution is lower than the freezing point of pure . On the other hand, when of sodium chloride are dissolved in the same mass of , the freezing point of the solution is lower than the freezing point of pure . Calculate the van't Hoff factor for sodium chloride in . Be sure your answer has a unit symbol, if necessary, and round your answer to significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1



Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3
You know the right answer?
When 136g of glycine are dissolved in of a certain mystery liquid , the freezing point of the soluti...
Questions


Social Studies, 25.11.2021 15:00

History, 25.11.2021 15:00


Chemistry, 25.11.2021 15:00

English, 25.11.2021 15:00




Mathematics, 25.11.2021 15:00

English, 25.11.2021 15:00

Social Studies, 25.11.2021 15:00




Mathematics, 25.11.2021 15:00

Mathematics, 25.11.2021 15:00

Health, 25.11.2021 15:00


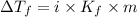
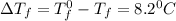 = Depression in freezing point
= Depression in freezing point
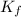 = freezing point constant
= freezing point constant 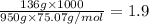
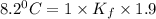
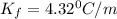
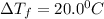 = Depression in freezing point
= Depression in freezing point