

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

You know the right answer?
Find the mass of 3.27 x 10^23 molecules of H2SO4. Use 3 significant digits
and put the units....
and put the units....
Questions


French, 20.10.2020 04:01





History, 20.10.2020 04:01






History, 20.10.2020 04:01

English, 20.10.2020 04:01


Arts, 20.10.2020 04:01



Mathematics, 20.10.2020 04:01

 .
. 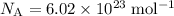 (three significant figures.)
(three significant figures.) ,
,  , and
, and  on a modern periodic table:
on a modern periodic table: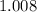 .
.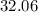 .
.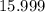 .
. atoms would be approximately
atoms would be approximately  grams on average.)
grams on average.) molecules without using any unit. Avogadro's Number
molecules without using any unit. Avogadro's Number  helps convert the unit of that count to moles.
helps convert the unit of that count to moles.  of these
of these  molecules.
molecules. 
 of such molecules.
of such molecules.  molecules. The value of the formula mass could be calculated using the relative atomic mass of each element:
molecules. The value of the formula mass could be calculated using the relative atomic mass of each element: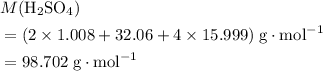 .
. of
of  .
.

