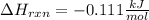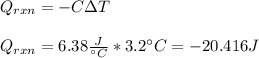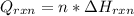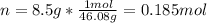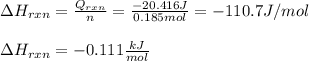Chemistry, 05.03.2021 23:00 sunpelt9993
An 8.5 g. Of ethanol (C2H5OH) placed in a constant volume calorimeter and the temperature rose to 3.2°C. Find the heat of reaction of the ethanol (MM =46.1 g/mol) in kJ/mol. The heat capacity of the calorimeter plus water is read as 6.38 J/°C

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3
You know the right answer?
An 8.5 g. Of ethanol (C2H5OH) placed in a constant volume calorimeter and the temperature rose to 3....
Questions


Spanish, 09.09.2020 22:01



Biology, 09.09.2020 22:01

Mathematics, 09.09.2020 22:01







Mathematics, 09.09.2020 22:01

English, 09.09.2020 22:01

Mathematics, 09.09.2020 22:01

Mathematics, 09.09.2020 22:01

Biology, 09.09.2020 22:01



Mathematics, 09.09.2020 22:01