
Chemistry, 05.03.2021 19:00 sydneepollitt32
Carbon dioxide and water react to form methanol and oxygen, like this:
2CO2(g) + 4H2O(g) → 2CH3OH(l) + 3O2 (g)
At a certain temperature, a chemist finds that a 7.5L reaction vessel containing a mixture of carbon dioxide, water, methanol, and oxygen at equilibrium has the following composition:
Compound Amount
CO2 3.28g
H2O 3.86g
CH3OH 1.51g
O2 2.80g
Required:
Calculate the value of the equilibrium constant Kc for this reaction. Round your answer to 2 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1
You know the right answer?
Carbon dioxide and water react to form methanol and oxygen, like this:
2CO2(g) + 4H2O(g) → 2CH3OH(l...
Questions




Mathematics, 06.09.2019 17:10

Mathematics, 06.09.2019 17:10







Mathematics, 06.09.2019 17:10








![\frac{[O_2]^3}{[CO_2]^2[H_2O]^4}](/tpl/images/1172/1518/03065.png)
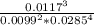 = 24x10³
= 24x10³

