Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
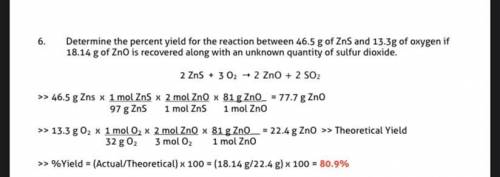
Determine the percent yield for the reaction between 46.5 g of
ZnS and 13.3 g of oxygen if 18.l4 g...
Questions


Mathematics, 24.06.2019 01:40



Spanish, 24.06.2019 01:40


Physics, 24.06.2019 01:40



Mathematics, 24.06.2019 01:40

History, 24.06.2019 01:40

English, 24.06.2019 01:40


Social Studies, 24.06.2019 01:40





Arts, 24.06.2019 01:40