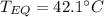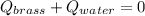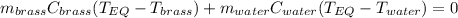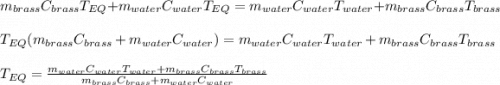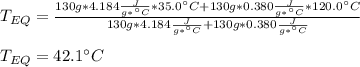Chemistry, 02.03.2021 14:00 LarryJoeseph
A 130 g sample of brass at 120.0 degrees Celsius is placed in a calorimeter cup that contains
130 g of water at 35.0 degrees Celsius. Disregard the absorption of heat by the cup and
calculate the final temperature of the brass and water. Specific heat of water = 4.18 J/gC,
specific heat of brass=0.380 J/gC. Attach your complete solution

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
A 130 g sample of brass at 120.0 degrees Celsius is placed in a calorimeter cup that contains
130 g...
Questions

Chemistry, 28.06.2019 02:00

Mathematics, 28.06.2019 02:00



Geography, 28.06.2019 02:00

Biology, 28.06.2019 02:00

Physics, 28.06.2019 02:00

Mathematics, 28.06.2019 02:00


Mathematics, 28.06.2019 02:00

History, 28.06.2019 02:00








English, 28.06.2019 02:00