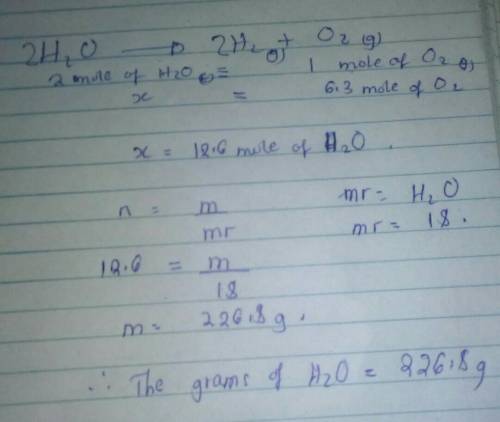Chemistry, 27.02.2021 14:00 PinkDivaGirl02
How many grams of H 2 O can be produced with 6.3 moles of O 2 ? IMPORTANT I NEED HELP Important please

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2
You know the right answer?
How many grams of H 2 O can be produced with 6.3 moles of O 2 ?
IMPORTANT I NEED HELP Important ple...
Questions

Chemistry, 04.11.2019 04:31

History, 04.11.2019 04:31

English, 04.11.2019 04:31




Chemistry, 04.11.2019 04:31


Chemistry, 04.11.2019 04:31



Biology, 04.11.2019 04:31






Mathematics, 04.11.2019 04:31


Chemistry, 04.11.2019 04:31