Chemistry, 24.02.2021 21:30 CameronVand21
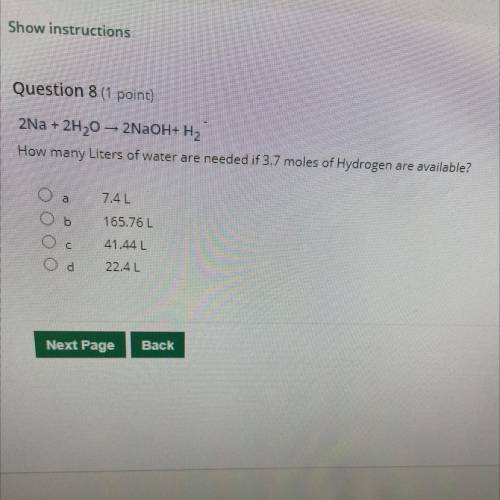
2Na + 2H20 – 2NaOH+H2 How many Liters of water are needed if 3.7 moles of Hydrogen are available?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
2Na + 2H20 – 2NaOH+H2
How many Liters of water are needed if 3.7 moles of Hydrogen are available?
<...
Questions


Health, 08.10.2019 08:00


English, 08.10.2019 08:00

Physics, 08.10.2019 08:00

History, 08.10.2019 08:00

Mathematics, 08.10.2019 08:00

Biology, 08.10.2019 08:00

History, 08.10.2019 08:00



History, 08.10.2019 08:00

Biology, 08.10.2019 08:00


Biology, 08.10.2019 08:00


English, 08.10.2019 08:00


Biology, 08.10.2019 08:00