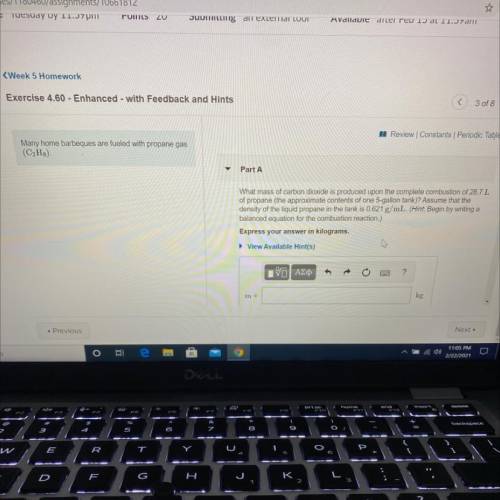
Many home barbeques are fueled with propane gas
(C3H8)
Part A: What mass of carbon dioxide is...

Chemistry, 23.02.2021 09:20 keilajohnson810
Many home barbeques are fueled with propane gas
(C3H8)
Part A: What mass of carbon dioxide is produced upon the complete combustion of 28.7L
of propane (the approximate contents of one 5-gallon tank)? Assume that the
density of the liquid propane in the tank is 0.621 g/mL.
Express your answer in kilograms.
Will give brainliest!

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
Questions



Mathematics, 16.03.2020 17:01



Mathematics, 16.03.2020 17:01

Mathematics, 16.03.2020 17:01


Mathematics, 16.03.2020 17:01

Physics, 16.03.2020 17:01

English, 16.03.2020 17:01




Mathematics, 16.03.2020 17:01





Business, 16.03.2020 17:02



