
Chemistry, 22.02.2021 18:50 Svetakotok
A 52.9g sample of brass, which has a specific heat capacity of 0.375·J·g^−1°C^−1, is put into a calorimeter (see sketch at right) that contains 100.0g of water. The temperature of the water starts off at 15.0°C. When the temperature of the water stops changing it's 18.4°C. The pressure remains constant at 1 atm. Calculate the initial temperature of the brass sample.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 23.06.2019 18:30
Which of the following describes a base? select all that apply. a. tastes sour b. tastes bitter c. highly reactive d. slippery feel
Answers: 2
You know the right answer?
A 52.9g sample of brass, which has a specific heat capacity of 0.375·J·g^−1°C^−1, is put into...
Questions

Mathematics, 28.09.2019 07:30

Biology, 28.09.2019 07:30


Mathematics, 28.09.2019 07:30


Biology, 28.09.2019 07:30






History, 28.09.2019 07:30


Mathematics, 28.09.2019 07:30


Mathematics, 28.09.2019 07:30

Mathematics, 28.09.2019 07:30

Social Studies, 28.09.2019 07:30

Spanish, 28.09.2019 07:30

Mathematics, 28.09.2019 07:30

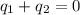
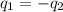
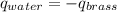
![m_{w}.c_{w}.\Delta T=-[m_{b}.c_{b}.\Delta T]](/tpl/images/1136/4104/e97cd.png)
![100.4.18.(18.4-15)=-[52.9.0.375.(18.4-T)]](/tpl/images/1136/4104/69ce0.png)


