
Chemistry, 22.02.2021 15:00 skylarrrrr7663
A chemist titrates 190.0 mL of a 0.8125 M ammonia (NH) solution with 0.3733 M HCl solution at 25 °C. Calculate the pH at equivalence. The pK, of
ammonia is 4.75.
Round your answer to 2 decimal places.
Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HCl solution added.
pH ?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3


Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
You know the right answer?
A chemist titrates 190.0 mL of a 0.8125 M ammonia (NH) solution with 0.3733 M HCl solution at 25 °C....
Questions

History, 05.07.2019 06:20



Social Studies, 05.07.2019 06:20

Spanish, 05.07.2019 06:20

Biology, 05.07.2019 06:20


Mathematics, 05.07.2019 06:20



History, 05.07.2019 06:20

English, 05.07.2019 06:20


Mathematics, 05.07.2019 06:20

Biology, 05.07.2019 06:20

Mathematics, 05.07.2019 06:20

History, 05.07.2019 06:20

Mathematics, 05.07.2019 06:20


Biology, 05.07.2019 06:20

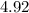 .
. 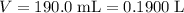 .
.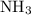 :
: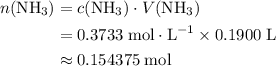 .
.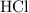 acid at a one-to-one ratio:
acid at a one-to-one ratio: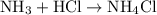 .
. 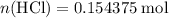 of
of 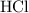 molecules would be required to exactly react with the
molecules would be required to exactly react with the 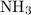 in the original solution and hence reach the equivalence point of this titration.
in the original solution and hence reach the equivalence point of this titration.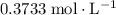
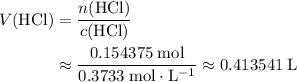 .
.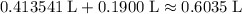 .
.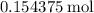 of
of 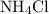 would be produced. Because
would be produced. Because 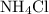 is a soluble salt, the solution would contain
is a soluble salt, the solution would contain 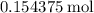 of
of 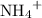 ions. The concentration of
ions. The concentration of 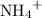 would be approximately:
would be approximately: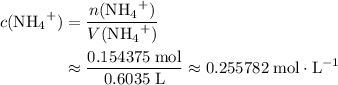 .
.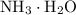 is a weak base, its conjugate
is a weak base, its conjugate 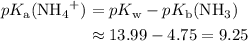 .
. 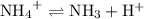 .
.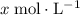 be the increase in the concentration of
be the increase in the concentration of 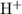 in this solution because of this reversible reaction. (Notice that
in this solution because of this reversible reaction. (Notice that 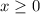 .) Construct the following
.) Construct the following  table:
table: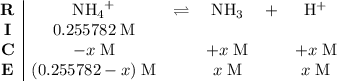 .
.![[{\rm {NH_4}^{+}}] \approx (0.255782 - x) \; \rm M](/tpl/images/1135/8062/3a08e.png) .Concentration of the conjugate of the weak acid:
.Concentration of the conjugate of the weak acid: ![[{\rm NH_3}] = x\; \rm M](/tpl/images/1135/8062/5c451.png) .Concentration of
.Concentration of ![[{\rm {H}^{+}}] \approx x\; \rm M](/tpl/images/1135/8062/2a607.png) .
.![\displaystyle \frac{[{\rm NH_3}] \cdot [{\rm H^{+}}]}{[{ \rm {NH_4}^{+}}]} = 10^{pK_\text{a}({\rm {NH_4}^{+}})}](/tpl/images/1135/8062/50d33.png) .
.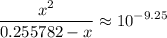
 . (Notice that the value of
. (Notice that the value of  is likely to be much smaller than
is likely to be much smaller than 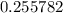 . Hence, the denominator on the left-hand side
. Hence, the denominator on the left-hand side 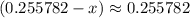 .)
.)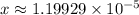 .
. .
.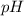 at the equivalence point of this titration would be:
at the equivalence point of this titration would be:![\begin{aligned}pH &= -\log_{10}[{\rm {H}^{+}}] \\ &\approx -\log_{10} \left(1.19929 \times 10^{-5}\right) \approx 4.92\end{aligned}](/tpl/images/1135/8062/8a0d9.png) .
.

