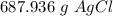Chemistry, 20.02.2021 05:00 Hcalhoun21
How many grams of silver chloride can be produced by reacting excess silver nitrate with 2.4 moles of zinc chloride? AgNO3 + ZnCl2 AgCl + Zn(NO3)2

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 22.06.2019 20:40
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
How many grams of silver chloride can be produced by reacting excess silver nitrate with 2.4 moles o...
Questions

Mathematics, 19.08.2019 02:10


History, 19.08.2019 02:10

Mathematics, 19.08.2019 02:10

Social Studies, 19.08.2019 02:10

Mathematics, 19.08.2019 02:10

Social Studies, 19.08.2019 02:10



History, 19.08.2019 02:10



Mathematics, 19.08.2019 02:10

Mathematics, 19.08.2019 02:10

Spanish, 19.08.2019 02:10


Mathematics, 19.08.2019 02:10

Biology, 19.08.2019 02:10


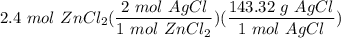 [DA] Multiply/Divide [Cancel out units]:
[DA] Multiply/Divide [Cancel out units]: