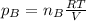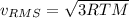Chemistry, 15.02.2021 19:40 mikehager4321
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon.
a. Which of the two gases exerts the greater partial pressure?
b. The molecules or atoms of which gas have the greater average velocity?
c. The molecules of which gas have the greater average kinetic energy?
d . If a small hole were opened in the flask, which gas effuses more quickly?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1
You know the right answer?
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon.
a. Whi...
Questions

Mathematics, 13.08.2020 19:01






Biology, 13.08.2020 19:01












Business, 13.08.2020 19:01