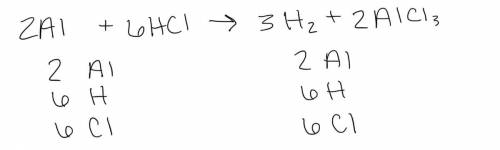Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
You know the right answer?
Balancing the following equation. Please use numerical answers
and use the number one instead of le...
Questions

Mathematics, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00



Physics, 15.04.2021 01:00

English, 15.04.2021 01:00


Arts, 15.04.2021 01:00


Computers and Technology, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00



Mathematics, 15.04.2021 01:00


Mathematics, 15.04.2021 01:00


Mathematics, 15.04.2021 01:00