
At 298 K, the Henry's law constant for oxygen is 0.00130 M/atm. Air is 21.0% oxygen.
1) At 298 K, what is the solubility of oxygen in water exposed to air at 1.00 atm?
2) At 298 K, what is the solubility of oxygen in water exposed to air at 0.89 atm?
3) If atmospheric pressure suddenly changes from 1.00 atm to 0.893 atm at 298 K, how much oxygen will be released from 4.70L of water in an unsealed container?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1
You know the right answer?
At 298 K, the Henry's law constant for oxygen is 0.00130 M/atm. Air is 21.0% oxygen.
1) At 298 K, w...
Questions


English, 10.06.2021 16:30






Biology, 10.06.2021 16:30

English, 10.06.2021 16:30




Mathematics, 10.06.2021 16:30

Mathematics, 10.06.2021 16:30

Mathematics, 10.06.2021 16:30

Mathematics, 10.06.2021 16:30

Mathematics, 10.06.2021 16:30

Mathematics, 10.06.2021 16:30


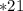 % of atm O2
% of atm O2 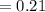 atm
atm
 mole / L
mole / L
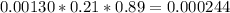 mole / L
mole / L
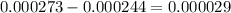
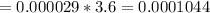 mole
mole 

