
Chemistry, 09.02.2021 15:50 fortwill05
What is the final, balanced equation that is formed by combining these two half reactions? 2 equations: first: upper C u right arrow upper C u superscript 2 plus, plus 2 e superscript minus. Second: upper N upper O subscript 3 superscript minus, plus 2 e superscript minus, plus 2 upper H superscript plus right arrow upper n upper O subscript 2 superscript minus plus upper H subscript 2 upper O.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
What is the final, balanced equation that is formed by combining these two half reactions? 2 equatio...
Questions



Arts, 23.04.2021 02:00

History, 23.04.2021 02:00


Biology, 23.04.2021 02:00

Health, 23.04.2021 02:00


Mathematics, 23.04.2021 02:00


Mathematics, 23.04.2021 02:00

Mathematics, 23.04.2021 02:00


Mathematics, 23.04.2021 02:00




Arts, 23.04.2021 02:00

Mathematics, 23.04.2021 02:00

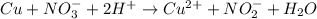
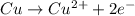 (1)
(1)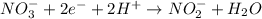 (2)
(2)

