
Chemistry, 09.02.2021 01:00 Josediego55
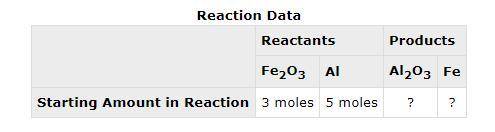
The following data was collected when a reaction was performed experimentally in the laboratory. Determine the maximum amount of Fe that was produced during the experiment. Explain how you determined this amount.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3
You know the right answer?
The following data was collected when a reaction was performed experimentally in the laboratory.
De...
Questions

Health, 24.02.2021 18:30


Mathematics, 24.02.2021 18:30

Mathematics, 24.02.2021 18:30

Mathematics, 24.02.2021 18:30

English, 24.02.2021 18:30

Mathematics, 24.02.2021 18:30

Chemistry, 24.02.2021 18:30

Mathematics, 24.02.2021 18:30



Mathematics, 24.02.2021 18:30

Mathematics, 24.02.2021 18:30


Mathematics, 24.02.2021 18:30

Mathematics, 24.02.2021 18:30

Mathematics, 24.02.2021 18:30


Geography, 24.02.2021 18:30

Mathematics, 24.02.2021 18:30




