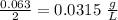Chemistry, 08.02.2021 19:00 amandasantiago2001
A chemistry student is given 2.00 L of a clear aqueous solution at 43.° C. He is told an unknown amount of a certain compound X is dissolved in the solution. The student allows the solution to cool to 25.° C. At that point, the student sees that a precipitate has formed. He pours off the remaining liquid solution, throws away the precipitate, and evaporates the water from the remaining liquid solution under vacuum. More precipitate forms. The student washes, dries and weighs the additional precipitate. It weighs 0.062 kg1) Using only the information above, can you calculate the solubility of X in water at 25 degrees C?2) If yes calculate it. Round answer to 2 significant digits

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1
You know the right answer?
A chemistry student is given 2.00 L of a clear aqueous solution at 43.° C. He is told an unknown amo...
Questions

Mathematics, 28.08.2019 12:30



Mathematics, 28.08.2019 12:30


Mathematics, 28.08.2019 12:30

Health, 28.08.2019 12:30


Mathematics, 28.08.2019 12:30

Geography, 28.08.2019 12:30




Chemistry, 28.08.2019 12:30



History, 28.08.2019 12:30

History, 28.08.2019 12:30


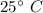 .
.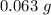 . They have a
. They have a 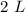 the solution, they may disregard the volume increases due to its precipitation. The intensity therefore is
the solution, they may disregard the volume increases due to its precipitation. The intensity therefore is