
Chemistry, 06.02.2021 03:40 addisonrausch
A reaction mixture in a 5.19 L flask at a certain temperature contains 26.9 g CO and 2.34 g H2. At equilibrium, the flask contains 8.65 g CH3OH. Calculate the equilibrium constant (Kc) for the reaction at this temperature.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 23.06.2019 06:40
4786 joules of heat are transferred to a 89.0 gramsample of an unknown material, with an initialtemperature of 23.0°c. what is the specific heat of thematerialif the final temperature is 89.5 °c?
Answers: 1

Chemistry, 23.06.2019 07:30
How do you interpret a chromagram for what mixtures contain?
Answers: 1
You know the right answer?
A reaction mixture in a 5.19 L flask at a certain temperature contains 26.9 g CO and 2.34 g H2. At e...
Questions

Computers and Technology, 13.12.2021 23:10

Mathematics, 13.12.2021 23:10




English, 13.12.2021 23:10

SAT, 13.12.2021 23:10






Mathematics, 13.12.2021 23:10

Mathematics, 13.12.2021 23:10


Mathematics, 13.12.2021 23:10


History, 13.12.2021 23:10


Arts, 13.12.2021 23:10

![\frac{[CH_3OH]_{eq}}{[CO]_{eq}([H_2]_{eq})^2}](/tpl/images/1098/4473/dbf7b.png) = 26.7
= 26.7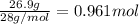
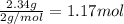
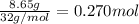
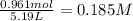
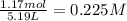
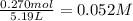
![[CO]_{eq}= 0.185 - 0.052 = 0.133 M\\\\\\[H_2]_{eq} = 0.225 - 2*0.052 = 0.121 M](/tpl/images/1098/4473/d17e2.png)
![K_c =\frac{[CH_3OH]_{eq}}{[CO]_{eq}[H_2_{eq}]^2} = 26.7](/tpl/images/1098/4473/cc90c.png)


