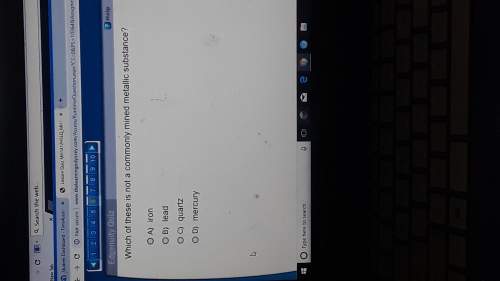
A student obtains a 1.00 mole sample of titanium metal and a 1.00 mole sample of silver metal. Which of the
following is a true statement about these two samples?
The titanium sample has more atoms than the silver sample.
O The titanium sample has more mass than the silver sample.
The silver sample has more atoms than the titanium sample.
The silver sample has more mass than the titanium sample.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
You know the right answer?
A student obtains a 1.00 mole sample of titanium metal and a 1.00 mole sample of silver metal. Which...
Questions


Social Studies, 07.06.2020 02:01


Biology, 07.06.2020 02:01


Chemistry, 07.06.2020 02:01


English, 07.06.2020 02:01


English, 07.06.2020 02:01

Mathematics, 07.06.2020 02:01









Biology, 07.06.2020 02:01