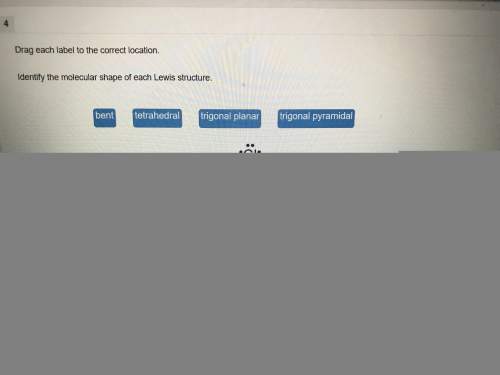
Chemistry, 02.02.2021 02:10 sonaihriley
A chemist is studying the rate of the Haber synthesis: N2 + 3H2 2NH3 Starting with a closed reactor containing 1.25 mol/L of N2 and 0.50 mol/L of H2, the chemist finds that the H2 concentration has fallen to 0.25 mol/L in 44 seconds. What is the N2 concentration after 44 seconds?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3


Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
A chemist is studying the rate of the Haber synthesis: N2 + 3H2 2NH3
Starting with a closed reactor...
Questions

Mathematics, 18.11.2020 21:50


English, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50



Mathematics, 18.11.2020 21:50


Mathematics, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50

Geography, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50


Mathematics, 18.11.2020 21:50



English, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50