
Chemistry, 01.02.2021 21:30 moomoofower
The first red (632.8 nm) Helium Neon gas laser was reported by White and Rigdon from Bell Labs in 1962. The laser was a discharge tube that was 1.2 m long with a 7 mm diameter. A He-Ne ratio of 10:1 was used with a total pressure of 0.00092 atm. This is a really low pressure with a really small amount of He and Ne. We want to try making a new laser with the same discharge tube with 5 mol Helium and 1 mol Neon. For the new laser with 5 mol He and 1 mol Ne, calculate the following quantities. Assume room temperature (298.15 K) and that the gasses are ideal.
a. What is the total volume (cm^3)?
b. What is the mole fraction of He?
c. What is the mole fraction of Ne?
d. What is the partial pressure of He (in atm)?
e. What is the partial pressure of Ne (in atm)?
f. What is the total pressure of the new laser tube (in atm)?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1


Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
The first red (632.8 nm) Helium Neon gas laser was reported by White and Rigdon from Bell Labs in 19...
Questions

History, 24.02.2021 15:40





Physics, 24.02.2021 15:50

English, 24.02.2021 15:50





Biology, 24.02.2021 15:50



Mathematics, 24.02.2021 15:50

Mathematics, 24.02.2021 15:50

Mathematics, 24.02.2021 15:50

Arts, 24.02.2021 15:50


Mathematics, 24.02.2021 15:50

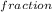 (He) = 0.8333
(He) = 0.8333 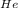 = 2648.68 atm
= 2648.68 atm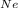 = 529.73 atm
= 529.73 atm

