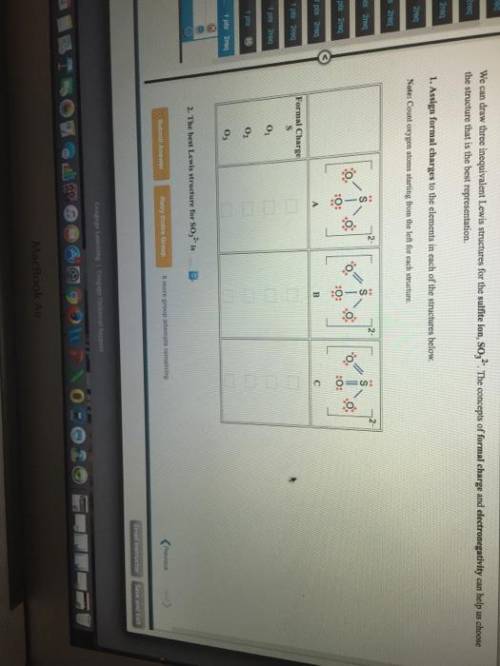
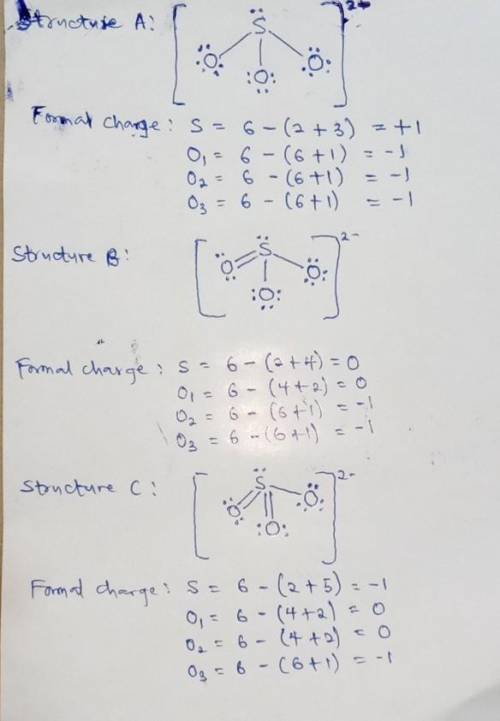
We can draw three inequivalent Lewis structures for the sulfite ion , SO32- . The concepts of formal charge and electronegativity can help us choose the structure that is the best representation. 1. Assign formal charges to the elements in each of the structures below. Note: Count oxygen atoms starting from the left for each structure. A B C Formal Charge S O1 O2 O3 2. The best Lewis structure for SO32- is

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1

Chemistry, 23.06.2019 12:50
What is the daughter nucleus produced a. when 217(at) undergoes alpha decay? b. when 103(mo) undergoes beta decay? c. when 188(hg) undergoes positron emission?
Answers: 1

Chemistry, 23.06.2019 15:00
The specific heat of a certain type of cooking oil is 1.75 cal/ (g c) how much heat energy is needed to raise the temperature of 2.67 kg of this oil from 23 c to 191 c
Answers: 1

Chemistry, 23.06.2019 16:10
Which of the following is the best name for a compound made from calcium and bromine? (cabr2) calcium bromide calcium dibromide monocalcium dibromide calcium bromine ii
Answers: 2
You know the right answer?
We can draw three inequivalent Lewis structures for the sulfite ion , SO32- . The concepts of formal...
Questions







Biology, 10.07.2019 12:30

Health, 10.07.2019 12:30

Health, 10.07.2019 12:30

Mathematics, 10.07.2019 12:30


Mathematics, 10.07.2019 12:30


Chemistry, 10.07.2019 12:30



Chemistry, 10.07.2019 12:30

History, 10.07.2019 12:30


Mathematics, 10.07.2019 12:30