Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
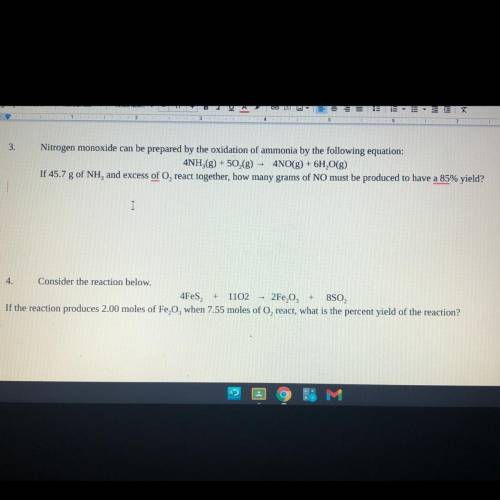
Consider the reaction below.
4Fes2 + 11O2 —> 2Fe2O3 + 8SO2
If the reaction produces 2.00 m...
If the reaction produces 2.00 m...
Questions




Mathematics, 18.03.2021 01:30


English, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Computers and Technology, 18.03.2021 01:30



Mathematics, 18.03.2021 01:30


English, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

History, 18.03.2021 01:30



Physics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30