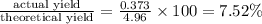Chemistry, 25.01.2021 18:00 MikeCrotch19251
2H2 (1) + O2(g) → 2H20 (g)
1. Find the limiting reactant if you start with 30.0 grams of hydrogen and 5.29 grams of oxygen.
2. The actual yield for H2O in the above reaction is 6.72 g, Determine the percent yield for the reaction
when 9.93 grams of hydrogen and excess oxygen react?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 23.06.2019 09:00
Which of the following are in a chemical family a. ca, sc, k b. cu, ag, au c. so, ge, sb
Answers: 1
You know the right answer?
2H2 (1) + O2(g) → 2H20 (g)
1. Find the limiting reactant if you start with 30.0 grams of hydrogen a...
Questions

English, 25.07.2021 23:50



Computers and Technology, 25.07.2021 23:50

Chemistry, 25.07.2021 23:50


Mathematics, 25.07.2021 23:50

English, 25.07.2021 23:50


English, 25.07.2021 23:50

Mathematics, 25.07.2021 23:50







Mathematics, 25.07.2021 23:50


Mathematics, 25.07.2021 23:50

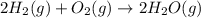
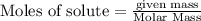
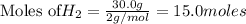
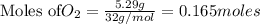
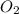 require = 2 moles of
require = 2 moles of 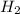
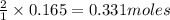 of
of 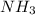
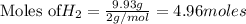
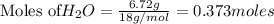
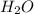
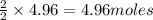 of
of