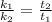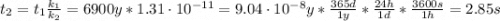Chemistry, 21.01.2021 22:50 DSUDLER5555
The activation energy for a reaction is changed from 184 kJ/mol to 59.0 kJ/mol at 600. K by the introduction of a catalyst. If the uncatalyzed reaction takes about 6900 years to occur, about how long will the catalyzed reaction take

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
The activation energy for a reaction is changed from 184 kJ/mol to 59.0 kJ/mol at 600. K by the intr...
Questions

Mathematics, 01.02.2021 21:00

Computers and Technology, 01.02.2021 21:00

Mathematics, 01.02.2021 21:00




Mathematics, 01.02.2021 21:00



English, 01.02.2021 21:00

Mathematics, 01.02.2021 21:00



Mathematics, 01.02.2021 21:00

History, 01.02.2021 21:00





Social Studies, 01.02.2021 21:00

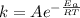
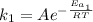 (1)
(1)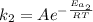 (2)
(2)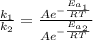
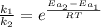
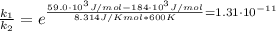
![k = \frac{\Delta [R]}{t}](/tpl/images/1053/9456/cc162.png)
![\frac{k_{1}}{k_{2}} = \frac{\Delta [R]/t_{1}}{\Delta [R]/t_{2}}](/tpl/images/1053/9456/eb896.png)