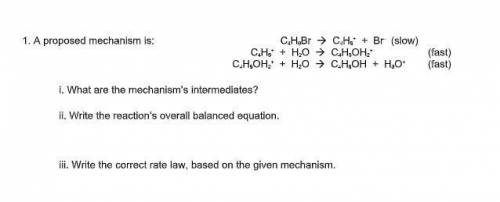
A proposed mechanism is:
C4H9Br --> C4H9^+ + Br^– (slow)
C 4H9^+ + H2O --> C4H9OH2^+ (f...

A proposed mechanism is:
C4H9Br --> C4H9^+ + Br^– (slow)
C 4H9^+ + H2O --> C4H9OH2^+ (fast)
C4H9OH2^+ + H2O --> C4H9OH + H3O^+ (fast)
i. What are the mechanism’s intermediates?
ii. Write the reaction’s overall balanced equation.
iii. Write the correct rate law, based on the given mechanism.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 23.06.2019 05:00
110 g of water (specific heat = 4.184 j/g c) and 100 g of a metal sample (specific heat = 0.397 j/g c) are heated from 25 degrees c to 75 degrees c. which substance required more thermal energy?
Answers: 1
You know the right answer?
Questions

English, 15.09.2021 20:30



English, 15.09.2021 20:30




Social Studies, 15.09.2021 20:30






English, 15.09.2021 20:30


Mathematics, 15.09.2021 20:30


Physics, 15.09.2021 20:30

Biology, 15.09.2021 20:30



