How many moles of NO2 are there in 5.10 x 10 18*molecules of NO2?
...

Chemistry, 19.01.2021 08:50 abadir2008
How many moles of NO2 are there in 5.10 x 10 18*molecules of NO2?
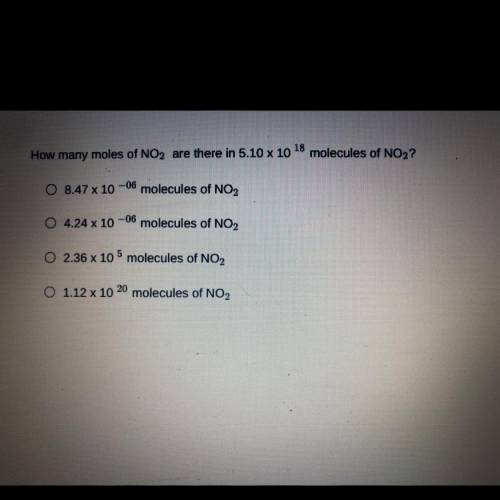

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
Questions


History, 21.05.2021 18:00




Mathematics, 21.05.2021 18:00

Mathematics, 21.05.2021 18:00


Geography, 21.05.2021 18:00

Biology, 21.05.2021 18:00

Mathematics, 21.05.2021 18:00


Mathematics, 21.05.2021 18:00


English, 21.05.2021 18:00


Computers and Technology, 21.05.2021 18:00





